New Stryker Hip Implant Recall Expected
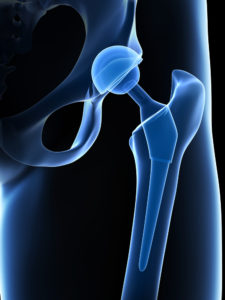
In 2012, Stryker Corp. recalled two of its metal on metal hip implant systems- the Rejuvenate Modular and ABG II Modular-Neck Hip Stems. All-metal implants account for one out of every 3 hip replacements. These devices have been abandoned by device manufacturers after complications arose from the metal components rubbing together and creating tiny metallic debris that causes serious damage to tissue and muscle.
In late 2014, Stryker announced it set aside $1.4 billion to offer settlements to those patients with the defective hip implants who had to undergo revision surgery. The average payout to victims is around $300,000.
If you or someone you know has experienced complications from a hip replacement from a defective implant, please contact my office for help.


